Image credit: Shutterstock
A net-zero economy will require marketable, large-scale, and energy-efficient solutions to capture carbon dioxide (CO2). For the greatest effects, these would be implemented at high-emitting locations, such as power plants, metal, cement, and chemical refineries. However, this is more easily said than done.
Despite the challenges associated with developing feasible and practical solutions, researchers at the University of Calgary have come up with a promising metal organic framework (MOF) called CALF-20 that can selectively capture CO2 in large quantities and is already finding application in industry.
The trouble with carbon capture
One of the most promising strategies in the race for carbon neutrality is carbon capture and sequestration. Typically, captured liquid CO2 is stored in underground sites or chemically trapped in mineralized carbonates or bicarbonates. However, the difficulty in this process lies not in how the notorious greenhouse gas would be stored, but how it would be captured.
This is especially true when much of known carbon-capture materials are limited by the amount of energy they need to regenerate the carbon capture material and CO2. Additional parameters for CO2 adsorbents must also be considered, such as its adsorption capacity and how it would fare in the presence of contaminant gases like nitrogen, oxygen, water, and acidic NOx and SOx in waste gas streams, where carbon capture would produce the greatest effects.
In this context, while amine liquids, hydroxide solutions, and organic solvents can physically and/or chemically absorb CO2, the regeneration and recycling processes for these adsorbents are often too energy intensive and susceptible to contamination, decomposition, system corrosion, and escape into the environment.
Alternatively, solid adsorbents overcome some of these issues, but at a cost. For example, solids that bind CO2 often require more energy to separate the CO2 from the absorbent. Moreover, they can be rendered ineffective by competitive binding from any of the other gases mentioned above. Thus, the thermodynamics and kinetics of adsorption and desorption are also key considerations in the effective engineering of capture and regeneration processes.
CALF-20
CALF-20 is an intricate layered crystal composed of zinc, triazolate, and oxalate arranged in a 3D structure filled with nanometer-scale holes for CO2 capture. The pores weakly yet preferentially bind CO2 over water by preventing the formation of hydrogen-bonds found in the latter. This means that water will not be able to stick to the material’s surface and block CO2 adsorption.
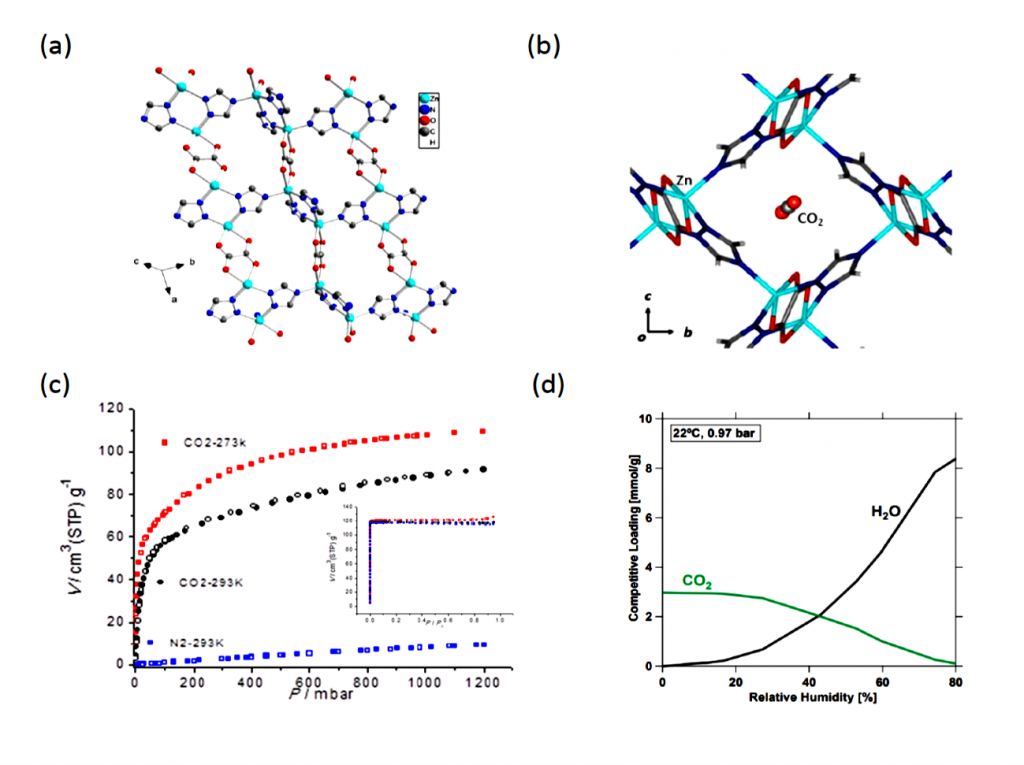
CALF-20 is now being used by Canadian company Svante in their revolutionary carbon capture process. A gram of this material has a massive surface area of 500 square meters, displays selective CO2 physisorption at high capacities, and only requires modest amounts of energy to remove CO2. Combined with its cheap starting materials and highly scalable single-step synthesis, the material boasts exceptional durability under long-term exposure to steam and wet NOx and SOx acidic gases over the 2000-hours of continuous testing. Furthermore, the regenerated CO2 was able to consistently meet the US Department of Energy’s purity target of 95%.
“It is difficult to perceive all the hurdles to span from molecular scale to field demonstration,” said CALF-20 inventor George Shimizu, professor in the Department of Chemistry at the University of Calgary. “It is critical to do all meaningful tests on your material to de-risk the financial, infrastructure, and time investment by those further up the technology readiness level ladder.”
With CALF-20’s implementation at Svante, the company’s vision is to make commercial gigaton-scale carbon capture a reality by 2050. They have developed rotating CO2 capture machines which use an adsorption-desorption cycle based on pressure and temperature gradients between waste gas and CO2 streams to efficiently transfer CO2 between the two lines. The cycles occur extremely quickly (a mere 60 seconds!) within the confines of a single cylindrical drum filled with rolls of CALF-20 sheets. CALF-20 is currently employed in a 1 tonne per day capture demonstration at a cement plant in Richmond, British Columbia.
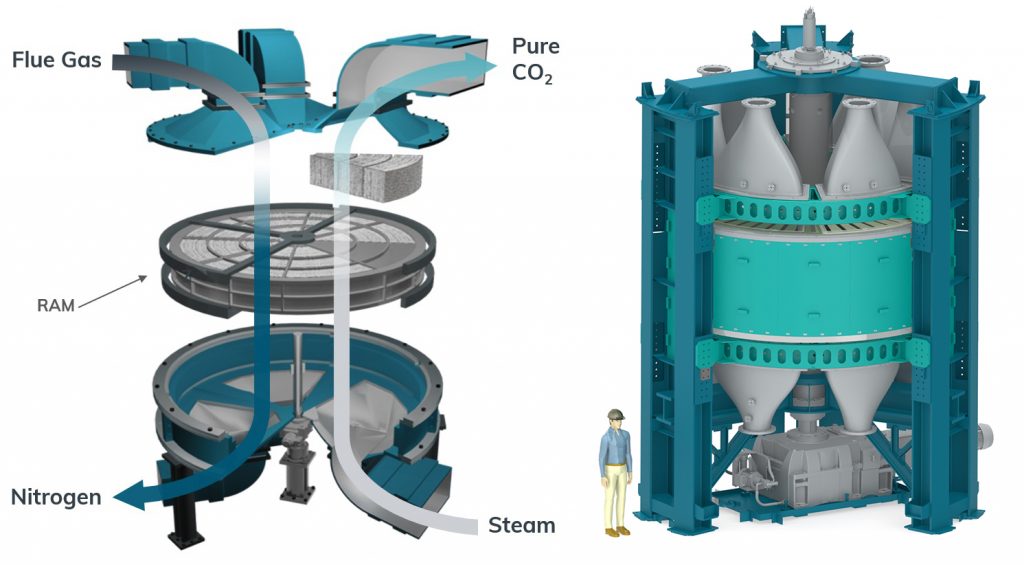
“This [technology] is crucial if we want to fight climate change and be able to remove gigatons of CO2,” said vice president of research and development at Svante, Pierre Hovington. “Universities and industries need to team together and work as fast as possible!”
These machines have widely scalable CO2 capture capacities, ranging from 30-1000 tons per day. This is equivalent to a sphere of pure CO2 with a diameter of 30 to 100 meters. Eventually, the company will aim to produce systems capable of capturing and storing 3000 tons, equivalent to a sphere nearly 150 meters in diameter, per day. They also foresee the addition of thousands of plants at high emission sources to meet the ICPP gigaton CO2 target by 2050-2070.
With this promising example, who knows what other developments the world of green technology can hold?
Written by: Geoff Ozin1, Jessica Ye, and Eric Bauchman2
1Solar Fuels Group, University of Toronto, g.ozin@utoronto.ca, [email protected] Website: www.solarfuels.utoronto.ca
2ebachman@co2rail.com, Website: www.Co2rail.com
Reference: Jian-Bin Lin, et al., A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture, Science (2021). DOI: 10.1126/science.abi7281

















